The HM Digital HM AP-2 AquaPro Water Quality Tester measures electrical conductivity over the range 0-9999 µS (µS/cm). C.B. has encountered days on Governor’s Brook, when it exceeded the max. I suggested the following procedure in such instances:
If you get one of those super high values again, here is a procedure we can use to estimate the actual value.
Either carry a small container, e,g, 200 mL with you, or go back with one and fill the container with the stream water. (Measure EC on site again if you go back to do it). At home, leave the container over night (or longer, no hurry; leave the cap on to stop evaporation) to allow it to come to constant,(room) temperature,
Fill another larger container (e.g. 1 liter) with tap water and allow it also to come to room temperature.
Then, Pour the stream water in a glass*. Measure the EC and Temp. Record EC & Temp.
Fill the same container with the tap water (left overnight), add to the glass, swirl to mix and measure EC again. Do this until you get two values in succession below 10,000, record those values and the temperatures.
Also, separately, measure the EC and Temperature of the Tap water left overnight.
So we have these values:
(i) EC & Temp measured on site e.g. maxed at 10,000
(ii) EC and Temp of tap water allowed to come to room temperature, e.g. EC 90, temp 16 deg (my tap water at home is around this value)
(iii) EC and Temp of stream water allowed to come to room temperature, e.g. EC 10,000, temp 16 deg
(iv) EC of stream water after adding one volume of Tap Water : e.g. 10,000 (still maxed out)
(v) EC of stream water after adding two volumes of Tap Water, e.g. 7500 Temp 16 degrees
(vi) EC of stream water after adding three volumes of Tap Water, e.g. 5800 Temp 16 degrees
Actually EC of original sample, calculation
X is unknown EC value we want to estimate
Y is the EC of the tap water (ii)
V is the number of container volumes of tap water you have added to the original sample
Z is the EC value (from v, or vi)
Formula X= Z*V + Z – V*Y
So with the above example, the estimate based on (v) is
X= 2*7500 +7500 – 90 = 22410
and the estimate based on vi is
X= 3*5800 +5800 – 90 = 23110
The estimated values should be close, but will prob be slightly different because the meter calibration in practice changes slightly with concentration, and they apply a single correction factor. So in this case, we estimate the real value as (22410+23110)/2= 22,760 at 16 degrees
If you want, just record the values and I can make the calculation, but you might enjoy the fun of doing it and discovering just how salty that water is! Regardless, put all results on a piece of paper, photo it and send it to me to check calculations.
*You may have to fiddle a bit with the glass size; it should allow a measurement with proper immersion with the first addition from the container…. if it gets too full with the additions, you can put it all in a larger glass, or pot or whatever. Also, between each measurement, wash electrodes with tap water, shake, and touch with paper towel to remove any remaining water.
All clear as mud?
Evidently not. On Jn 21, C.B. sent this to me commenting ‘How does this look:
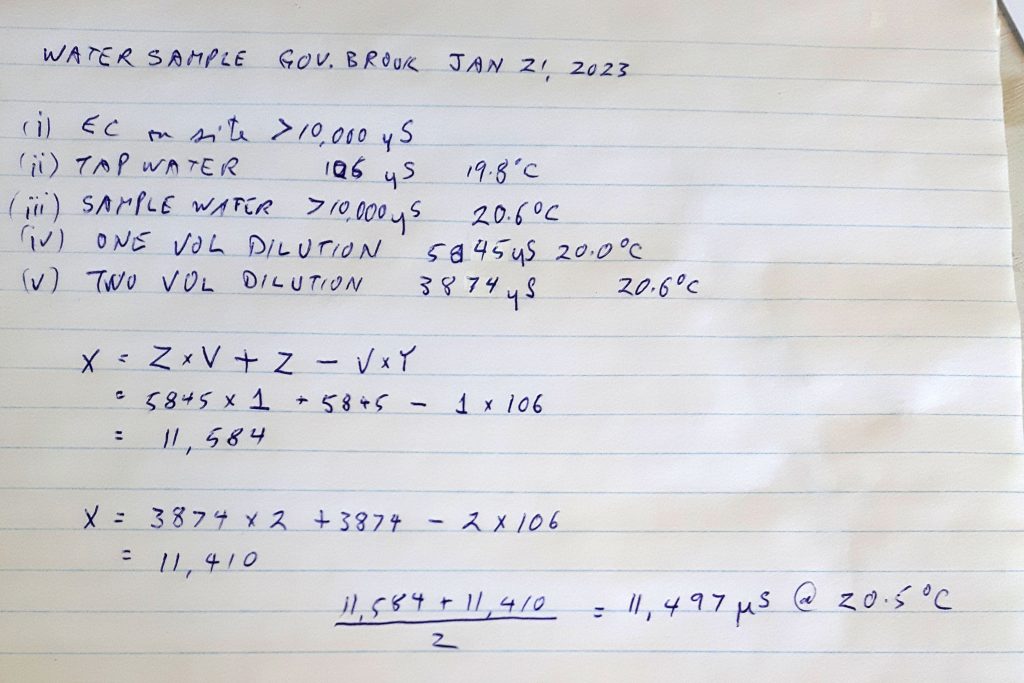
Just Fine! And pretty dam salty (Full strength seawater has an EC value of approx. 50,000.)
